Alpha radiation...
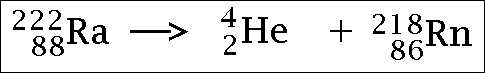
(the He part is the alpha particle)
Beta radiation...
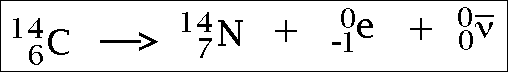
(the e part is the beta particle)
Gamma radiation...
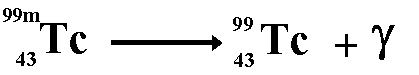
A blog covering and explaining the Edexcel IGCSE Physics specification for the 2016 summer exams. If you are doing just double science, you do not need to learn the stuff for paper two, if you are doing triple you will need to learn all (GOOD LUCK!) I have separated the papers to make files easier to find. Hope it helps :)
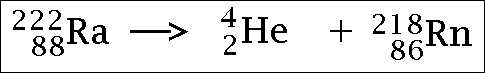
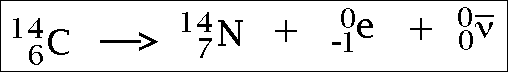
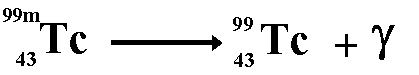
I don't understand the last part with the gamma rays.
ReplyDeleteWhat does the 'm' in "99m" represent and whys isn't there anymore after the gamma ray has been emitted.
Hi!! Sorry I didn’t put up a very clear picture of gamma radiation (will add a better one when I get home). 99m is just an isotope of Tc. We don’t need to know what it stands for - all that is important is that we realise that with gamma radiation we don’t lose any protons or neutrons (as apposed to with alpha and beta radiation).
Delete