The mass number is the number of protons and neutrons in a nucleus
Isotopes are atoms with the same number of protons (so the same atomic/proton number) but a different number of neutrons (so different mass number). However, because it is the number of protons in an atom that determine its element, the element is the same. That was confusing, here is an example that may help...
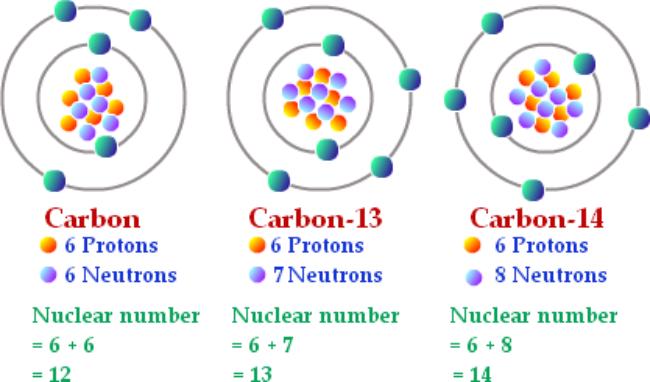
For example, carbon-13 and carbon-14 are both isotopes of carbon, as they all contain the same amount of protons but each have a different amount of neutrons.
Image source: experiment.com
It should read nucleon number, not nuclear number
ReplyDelete