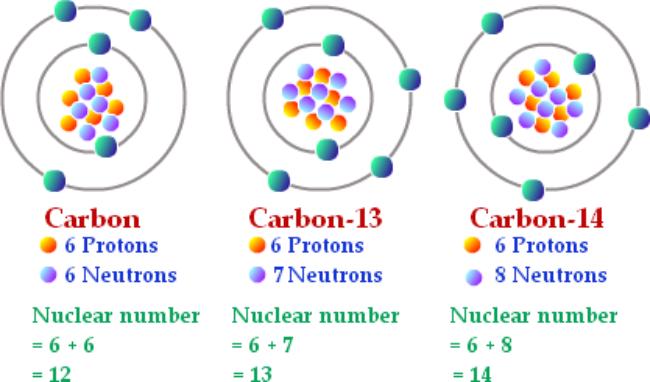
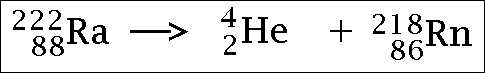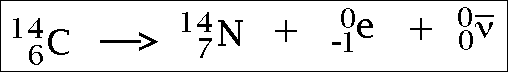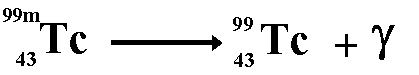Renewable
- hydro-electric power: very expensive, produces small amounts of electricity
- Solar panels: produce lots of energy, rely on weather
- Nuclear reactors/nuclear power: produces lots of energy quickly/easily, produces lots of harmful waste products
- Wind power: visual pollution; produces small amounts of electricity for space and effort in comparison to other methods, makes use of what we have (e.g. a 'free' energy source)
Non-renewable
Fossil fuels: finite source (will run out one day), releases CO2.