Geiger-Muller detectors beep when ionizing radiation is detected. The faster the beep, the more radiation. NOTE: there will always be slow, steady beeps due to background radiation.
Photographic film is white. It absorbs radiation and turns black. NOTE: photographic film is used for X-rays, the bone part is white as the radiation is absorbed by the bone therefore the film behind it s not exposed to the radiation. Everywhere is the film where there is no bone is black as there is nothing blocking the radiation from getting through.
A blog covering and explaining the Edexcel IGCSE Physics specification for the 2016 summer exams. If you are doing just double science, you do not need to learn the stuff for paper two, if you are doing triple you will need to learn all (GOOD LUCK!) I have separated the papers to make files easier to find. Hope it helps :)
Showing posts with label section 7. Show all posts
Showing posts with label section 7. Show all posts
Sunday, 22 May 2016
Thursday, 19 May 2016
7.19 understand that a chain reaction can be set up if the neutrons produced by one fission strike other U-235 nuclei
During nuclear fission, a slow-moving neutron gets absorbed by the nucleus of a U-235 atom. When this occurs, the atom splits into 2 daughter nuclei, whilst also releasing a small number of nuclei. If these nuclei his other uranion-235 atoms, these atoms will split and release more nuclei. The process will repeat. this is known as a chain reaction.
7.18 understand that the fission of U-235 produces two daughter nuclei and a small number of neutrons
In nuclear fission, a slow moving neutron gets absorbed by the nucleus of an U-235 atom. This causes the atom to split. The nucleus will split into two smaller 'daughter' nuclei and will also 'spit out' a small number of neutrons.
NOTE: When uranium-235 splits into two daughter cells, these cells will be radioactive as they will have the 'wrong' number of neutrons in them. They will also be lighter elements than uranium.
NOTE: When uranium-235 splits into two daughter cells, these cells will be radioactive as they will have the 'wrong' number of neutrons in them. They will also be lighter elements than uranium.
7.17 understand that a nucleus of U-235 can be split (the process of fission) by collision with a neutron, and that this product releases energy in the form of kinetic energy of the fission products
Nuclear power stations get there energy from a process of splitting atoms by collision with a neutron(as this releases energy). This is how...
If a slow moving neutron will get absorbed by an atom of uranium-235 (it will absorb into the nucleus). When this happens, the U-235 nucleus will split and spits out a small number of neutrons as it does.
This process releases energy (kinetic) and is converted into heat energy in the reactor by collisions with other atoms.
If a slow moving neutron will get absorbed by an atom of uranium-235 (it will absorb into the nucleus). When this happens, the U-235 nucleus will split and spits out a small number of neutrons as it does.
This process releases energy (kinetic) and is converted into heat energy in the reactor by collisions with other atoms.
Sunday, 8 May 2016
7.15 describe the results of Geiger and Marsden's experiments with gold foil and alpha particles
In an attempt to disprove the plum pudding model, Geiger and Marsden set up an experiment in which they positioned a sheet of gold foil in a circle of zinc sulphide screen. They then aimed alpha particles at a sheet of thin gold foil. They concluded that most of the alpha particles went straight through the foil, and gave a tiny flash (a scintillation) when they hit the zinc sulphide screen. However, some of the alpha particles were deflected at 90º to the direction they were traveling, and some came straight back. This concluded that inside an atom there must be positively charged nuclei which repel the alpha particles (this is why they 'bounce off' at different directions).
7.14 describe the dangers of ionising radiations, including: radiation can cause mutations in living organisms radiation can damage cells and tissue the problems arising in the disposal of radioactive waste and describe how the associated risks can be reduced.
Radiation can damage cells and tissue and mutations
Beta and gamma radiation are basically unharmful to humans as they can penetrate right out of the body. However, if alpha radiation gets inside the body, it can not escape as it cannot pass through human skin, therefore it can cause much damage. It collides with molecules, ionising them which will damage (and sometimes destroy) the molecule.
If the source is at a lower radiation, less damage will be done. For example, it can cause mutations, which can then divide uncontrollable, leading to serious medical conditions such as cancer.
If the source is at a high dose, the cells tend to be killed. This can lead to radiation sickness if a large part of your body is affected at the same time.
NOTE: The extent of the effects depends on how much exposure you have to the radiation and how much energy it has (e.g. how many half-lives has it lived, like does it still have lots of activity or has it already expelled lots and lots).
However, although radiation can cause cancer, it can also be used to treat cancer. If a patient is given a high dose of gamma rays (directed at the cells in the tumour), this can kill those specific cells without harming many others.
The problems arising in the disposal of radioactive waste and describe how the associated risks can be reduced
Low-level waste from places such as hospitals and nuclear power stations (e.g clothing sonf syringes) can be easily disposed of by burying them in landfill sites.
However, high-level waste is very dangerous as it has a very long half-life, so can stay radioactive for a super long time (like 10s of 1000's of years). This waste is often sealed in glass blocks which are sealed in metal canisters which are buried deep underground.
NOTE: This is hard to do as the site must be 'geologically stable' meaning no earthquakes etc as this could cause leakages... which means we die, basically.
Beta and gamma radiation are basically unharmful to humans as they can penetrate right out of the body. However, if alpha radiation gets inside the body, it can not escape as it cannot pass through human skin, therefore it can cause much damage. It collides with molecules, ionising them which will damage (and sometimes destroy) the molecule.
If the source is at a lower radiation, less damage will be done. For example, it can cause mutations, which can then divide uncontrollable, leading to serious medical conditions such as cancer.
If the source is at a high dose, the cells tend to be killed. This can lead to radiation sickness if a large part of your body is affected at the same time.
NOTE: The extent of the effects depends on how much exposure you have to the radiation and how much energy it has (e.g. how many half-lives has it lived, like does it still have lots of activity or has it already expelled lots and lots).
However, although radiation can cause cancer, it can also be used to treat cancer. If a patient is given a high dose of gamma rays (directed at the cells in the tumour), this can kill those specific cells without harming many others.
The problems arising in the disposal of radioactive waste and describe how the associated risks can be reduced
Low-level waste from places such as hospitals and nuclear power stations (e.g clothing sonf syringes) can be easily disposed of by burying them in landfill sites.
However, high-level waste is very dangerous as it has a very long half-life, so can stay radioactive for a super long time (like 10s of 1000's of years). This waste is often sealed in glass blocks which are sealed in metal canisters which are buried deep underground.
NOTE: This is hard to do as the site must be 'geologically stable' meaning no earthquakes etc as this could cause leakages... which means we die, basically.
7.13 describe the uses of radioactivity in medical and non-medical tracers, in radiotherapy, and in the radioactive dating of archaeological specimens and rocks
Medical tracers
This method is used for doctors to find out whether a persons organs are working as they should be. Alpha can not be used as it does not penetrate human skin and is strongly ionising, but beta and gamma can be used at it will penetrate human skin and body tissue.
The process...
- A source which emits beta or gamma radiation is injected or swallowed.
- The source moves around the body and the radiation will penetrate the body tissues and can be detected externally by a radiographer with a detector
- A computer converts the reading to a screen display which shows where the radiation is coming from
NOTE: The radioactive source they use would have to have a short half-life so it does not damage the person.
Non-medical tracers
Industrial tracers can be used for looking for things like leaks in underground pipes (using a tracer you would not need to dig out the pipe to find the leak, it's just a little bit less hassle).
The process...
- Put a gamma source into the pipe and let it flow through the pipe. Detect where the radiation goes with a detector above ground (follow it)
- When you reach the point where there is a hole in the pipe, there will b a much larger reading of radiation on the detector as lots of radiation will have escaped.
NOTE: A gamma source must be used, as beta or alpha would be stopped by the earths rocks and there would be a very little (if any) reading. It should also have a short half-life as it could cause damage if it stays/collects somewhere (think Chernobyl and Fukushima, but on a smaller scale)
Radioactive dating
Radioactive dating enables archaeologists to accurately work out the age of rocks, fossils and archaeological specimens (for example, Egyptian mummys)
If you know the half-life and amount of radioactive isotope in a sample, you can work out how long it has been around.
By comparing the activity level of an archaeological sample to a sample of living tissue, you can work out the amount of Carbon-14 half-lives that have passed (for example). This can give you an idea of how long ago the sample was living/died.
NOTE: an alternative is to look at the ratio of Carbon-14 to Carbon-12 as this is fixed in living materials, so by comparing the ratio in a living and non living sample you can estimate the age of the sample.
This method is used for doctors to find out whether a persons organs are working as they should be. Alpha can not be used as it does not penetrate human skin and is strongly ionising, but beta and gamma can be used at it will penetrate human skin and body tissue.
The process...
- A source which emits beta or gamma radiation is injected or swallowed.
- The source moves around the body and the radiation will penetrate the body tissues and can be detected externally by a radiographer with a detector
- A computer converts the reading to a screen display which shows where the radiation is coming from
NOTE: The radioactive source they use would have to have a short half-life so it does not damage the person.
Non-medical tracers
Industrial tracers can be used for looking for things like leaks in underground pipes (using a tracer you would not need to dig out the pipe to find the leak, it's just a little bit less hassle).
The process...
- Put a gamma source into the pipe and let it flow through the pipe. Detect where the radiation goes with a detector above ground (follow it)
- When you reach the point where there is a hole in the pipe, there will b a much larger reading of radiation on the detector as lots of radiation will have escaped.
NOTE: A gamma source must be used, as beta or alpha would be stopped by the earths rocks and there would be a very little (if any) reading. It should also have a short half-life as it could cause damage if it stays/collects somewhere (think Chernobyl and Fukushima, but on a smaller scale)
Radioactive dating
Radioactive dating enables archaeologists to accurately work out the age of rocks, fossils and archaeological specimens (for example, Egyptian mummys)
If you know the half-life and amount of radioactive isotope in a sample, you can work out how long it has been around.
By comparing the activity level of an archaeological sample to a sample of living tissue, you can work out the amount of Carbon-14 half-lives that have passed (for example). This can give you an idea of how long ago the sample was living/died.
NOTE: an alternative is to look at the ratio of Carbon-14 to Carbon-12 as this is fixed in living materials, so by comparing the ratio in a living and non living sample you can estimate the age of the sample.
7.12 use the concept of half-life to carry out simple calculations on activity
Okay so, this may be a bit confusing but here goes, this is best to show with an example...
E.g. the activity of a radioisotope is 640 Bq. Two hours later it has fallen to 40 Bq. Find the half-life of the sample.
All you have to do is keep dividing 640 until you reach 40... sort of.
Initial Bq is 640
After one half-life 640 / 2 = 320
After two half-lives 320 / 2 = 160
After three half-lives 160 / 2 = 80
After four half-lives 80 / 2 = 40
Okay so we made it to 40 in 4 half-lives, this means it took 4 half-lives for the activity to drop from 640 to 40, which took two hours, meaning 2 hours represents 4 half-lives, meaning each half-life is 30 minutes. So the half-life of this sample is 30 minutes
Answer = 30 minutes.
Example source: CGP
7.11 understand the term 'half-life' and understand that it is different for different radioactive isotopes
Definition for exams: half-life is the time taken for half of the radioactive atoms now present to decay.
It is very useful as there is a problem measuring how quickly the activity drops off for some isotopes, as they can last millions of years, so the half-life is used to measure how quickly activity falls off.
It is different for different isotopes as each isotope has a different amount of activity to expel. A short half-life means the activity fall quickly, because lots of the nuclei is decaying quickly (the half-life is short as it does not take up much time). A long half-life means the activity falls more slowly because most of the nuclei don't decay for a long time (the half-life is long as it takes a very very long time).
It is very useful as there is a problem measuring how quickly the activity drops off for some isotopes, as they can last millions of years, so the half-life is used to measure how quickly activity falls off.
It is different for different isotopes as each isotope has a different amount of activity to expel. A short half-life means the activity fall quickly, because lots of the nuclei is decaying quickly (the half-life is short as it does not take up much time). A long half-life means the activity falls more slowly because most of the nuclei don't decay for a long time (the half-life is long as it takes a very very long time).
Saturday, 7 May 2016
7.10 understand that the activity of a radioactive source decreases over a period of time and is measures in becquerels
A simple way to think of this is that each time decay happens, a little bit more of the radioactive nucleus has disappeared. As the unstable nuclei disappear, the activity as a whole will decrease (as less radiation will be given out).
The amount of radiation given out is measures in becquerels.
The amount of radiation given out is measures in becquerels.
7.9 explain the sources of background radiation
Background radiation is just radiation that is everywhere but it is only at a low level so it doesn't harm humans :) It comes from...
- cosmic rays (from the sun)
-living things (there is a little bit of radioactive material in all living things - including you and me!)
- substances on Earth (e.g. air, food, soil, rocks, building materials)
NOTE: radiation can also occur due to human activity. For example, there is a very high amount of radiation surrounding the areas of Chernobyl and Fukushima since they are the scenes of nuclear power plant disasters
- cosmic rays (from the sun)
-living things (there is a little bit of radioactive material in all living things - including you and me!)
- substances on Earth (e.g. air, food, soil, rocks, building materials)
NOTE: radiation can also occur due to human activity. For example, there is a very high amount of radiation surrounding the areas of Chernobyl and Fukushima since they are the scenes of nuclear power plant disasters
7.7 understand how to complete balanced nuclear equations
To do this all you need to know is the atomic and mass numbers of the original isotope, and the atomic/mass numbers of the particle/ray being emitted (these can both easily be worked out). You then put them in an equation... it is best to demonstrate with a few pictures...
Alpha radiation...
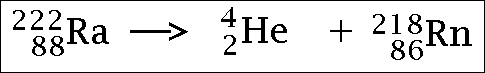
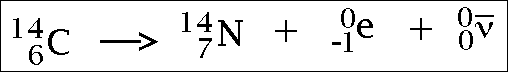
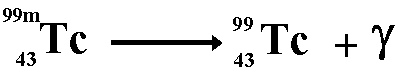
Alpha radiation...
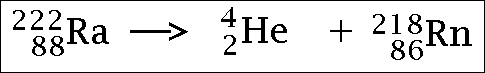
(the He part is the alpha particle)
Beta radiation...
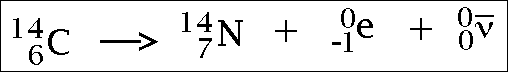
(the e part is the beta particle)
Gamma radiation...
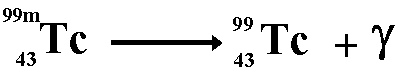
(the funny almost-Y shaped thing is the symbol for a gamma particle)
7.6 describe the effects on the atomic and mass numbers of a nucleus of the emission of each of the three main types of radiation
An alpha particle is made up of 2 protons and 2 neutrons, therefore, when an alpha particle is emitted, the proton number will decrease by 2 (as 2 protons have been released) and the mass number will decrease by 4 (as 4 nucleons will have been released).
A beta particle is comprised of one electron, this means that when a beta particle is emitted, the atomic number will increase by one.
There is no effect on an atoms atomic or mass numbers if a gamma ray is emitted. This is because it is comprised of energy, not protons/neutrons/electrons.
7.5 describe the nature of alpha and beta particles and gamma rays and recall that they may be distinguished in terms of penetrating power
NOTE: so I just wrote down about everything I could find/knew on alpha and beta particles and gamma rays, there is no need to learn all of it but I put it all down so you can choose which parts to learn, it may be an idea to learn 3-4 facts about each or at least the main distinguishing features.
Alpha particles are made up os 2 protons and 2 neutrons - this means they are 'big' and heavy, and move quite slowly too. Because of this, they don't penetrate far into materials, however, they are strongly ionising (due to there size). This means that they collide with a lot of atoms and knock off their electrons, this creates lots of ions (hence the term 'ionising'). Due to the fact they are made of 2protons and 2 neutrons, they have a positive electric charge, because of this, they are deflected by electric and magnetic fields. Because of their composition (of 2 neutrons ad 2 protons), if an atom emits an alpha particle, its atomic number will decrease by 2 (as 2 protons will have 'gone') and its mass number will decrease by 4 (as. altogether, 4 nucleons have 'gone').
Beta particles are comprised of an electron. A beta particle is just an electron which has been emitted from the nucleus of an atom when a neutron turns into a proton and an electron...bit confusing (it basically just helps to balance the charge). They are quite small and move fairly fast, therefore they penetrate materials quite far (but not super far) and are quite ionising too (but not as much as alpha particles). When a nucleus emits an electron the number of protons in the nucleus will increase by 1, meaning the atomic number increases by 1 but the mass number stays the same (i don't really understand this it... help). Because beta particles are negatively charged (as they are comprised on one electron), they are deflected by electric and magnetic fields (like alpha particles).
Gamma rays have no mass, they are just made of energy and therefore can penetrate far into materials without colliding into anything - this means they very weakly ionise, as they often pass through instead of hit atoms (although they eventually hit one, therefore they are not not-ionising). Since they have no mass, they have no charge, therefore they are not deflected by electric or magnetic fields. the emission of gamma rays has no effect on the proton or mass number of an isotope, as it has no mass. If a nucleus has too much energy, this is when a gamma ray is emitted (as it is just made up of energy, so emitting it will 'get rid' of all the excess energy).
NOTE: Gamma rays are only emitted after either alpha or beta particles (or maybe both), they are never emitted by themselves.
Since each type of radiation penetrates a material to different extents, it is easy to identify what it is by its penetrating power.
- If it is stopped by thin materials, such as paper or skin, it is alpha.
- If it is stopped by mediumish thickness materials, such as a sheet of aluminium, but can pass through paper, it is beta
- If it can pass through paper and aluminium but not thick lead, it is gamma.
Here is a diagram that may help for understanding purposes...

image source: gtcceis.gov
Alpha particles are made up os 2 protons and 2 neutrons - this means they are 'big' and heavy, and move quite slowly too. Because of this, they don't penetrate far into materials, however, they are strongly ionising (due to there size). This means that they collide with a lot of atoms and knock off their electrons, this creates lots of ions (hence the term 'ionising'). Due to the fact they are made of 2protons and 2 neutrons, they have a positive electric charge, because of this, they are deflected by electric and magnetic fields. Because of their composition (of 2 neutrons ad 2 protons), if an atom emits an alpha particle, its atomic number will decrease by 2 (as 2 protons will have 'gone') and its mass number will decrease by 4 (as. altogether, 4 nucleons have 'gone').
Beta particles are comprised of an electron. A beta particle is just an electron which has been emitted from the nucleus of an atom when a neutron turns into a proton and an electron...bit confusing (it basically just helps to balance the charge). They are quite small and move fairly fast, therefore they penetrate materials quite far (but not super far) and are quite ionising too (but not as much as alpha particles). When a nucleus emits an electron the number of protons in the nucleus will increase by 1, meaning the atomic number increases by 1 but the mass number stays the same (i don't really understand this it... help). Because beta particles are negatively charged (as they are comprised on one electron), they are deflected by electric and magnetic fields (like alpha particles).
Gamma rays have no mass, they are just made of energy and therefore can penetrate far into materials without colliding into anything - this means they very weakly ionise, as they often pass through instead of hit atoms (although they eventually hit one, therefore they are not not-ionising). Since they have no mass, they have no charge, therefore they are not deflected by electric or magnetic fields. the emission of gamma rays has no effect on the proton or mass number of an isotope, as it has no mass. If a nucleus has too much energy, this is when a gamma ray is emitted (as it is just made up of energy, so emitting it will 'get rid' of all the excess energy).
NOTE: Gamma rays are only emitted after either alpha or beta particles (or maybe both), they are never emitted by themselves.
Since each type of radiation penetrates a material to different extents, it is easy to identify what it is by its penetrating power.
- If it is stopped by thin materials, such as paper or skin, it is alpha.
- If it is stopped by mediumish thickness materials, such as a sheet of aluminium, but can pass through paper, it is beta
- If it can pass through paper and aluminium but not thick lead, it is gamma.
Here is a diagram that may help for understanding purposes...
image source: gtcceis.gov
Friday, 6 May 2016
7.4 understand that alpha and beta particles and gamma rays are ionising radiations emitted from unstable nuclei in a random process
Alpha and beta particles and gamma rays are emitted from unstable nuclei (in an attempt to make them more stable), this is known as radioactive decay. Radioactive decay is spontaneous and random, there is no (current) way of detecting when an unstable nucleus will decay and there is no way of speeding up/slowing down the process as it is completely unaffected by physical conditions (such as temperature of pH) or by and sort of chemical reactions/bondings.
NOTE: When a nucleus decays, it emits either alpha particles, beta particles or gamma rays (sometimes more than one kind, e.g alpha and gamma, or beta and alpha).
NOTE: When a nucleus decays, it emits either alpha particles, beta particles or gamma rays (sometimes more than one kind, e.g alpha and gamma, or beta and alpha).
Thursday, 5 May 2016
7.3 understand the terms atomic (proton)number, mass (nucleon) number and isotope
The atomic (or proton) number is just the amount of protons that are in an atom.
The mass number is the number of protons and neutrons in a nucleus
Isotopes are atoms with the same number of protons (so the same atomic/proton number) but a different number of neutrons (so different mass number). However, because it is the number of protons in an atom that determine its element, the element is the same. That was confusing, here is an example that may help...
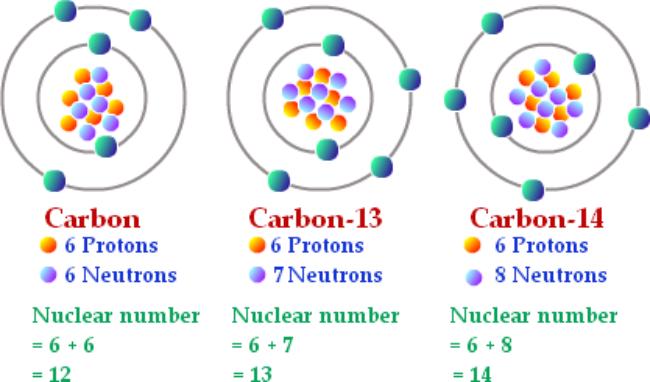
The mass number is the number of protons and neutrons in a nucleus
Isotopes are atoms with the same number of protons (so the same atomic/proton number) but a different number of neutrons (so different mass number). However, because it is the number of protons in an atom that determine its element, the element is the same. That was confusing, here is an example that may help...

For example, carbon-13 and carbon-14 are both isotopes of carbon, as they all contain the same amount of protons but each have a different amount of neutrons.
Image source: experiment.com
7.2 describe the structure of an atom in terms of protons, neutrons and electrons and use symbols such as 14 C to describe a particular nuclei
The structure of an atom is super simple when you break it down...
- all atoms contain a nucleus and electrons
- the nucleus of an atom is made up of protons and neutrons
- the total number of protons and neutrons is called the mass (or nucleon) number
- electrons surround the nucleus in electron shells
- the number of protons is the same as the number of electrons
- the number of protons/electrons is known as the atomic (or proton) number
From the mass number and proton number we can learn how many protons/neutrons an atom contains. For example, if an atom of carbon has a mass number of 14 and a proton number of 6, we know that there must be 6 protons in the nucleus. To find out how many neutron are in the shell, just minus the proton number from the mass number... 14 - 6 = 8, therefore there are 8 neutrons in this isotope of carbon.
7.1 use the following units: becquerel (Bq), centimetre (cm), hour (h), minute (min), second (s)
Becquerel, (Bq), measure of radioactivity
centimetre, (cm), measure of distance
hour, (h), measure of time
minute, (min), measure of time
second, (s), measure of time
centimetre, (cm), measure of distance
hour, (h), measure of time
minute, (min), measure of time
second, (s), measure of time
Subscribe to:
Posts (Atom)